A karyotype describes all the chromosomes of a cell, usually visualised as a systemised arrangement of chromosome pairs, in descending order of size.
It is used to analyze the number and structure of all the chromosomes and provides a low-resolution genome-wide screen for chromosomal abnormalities.
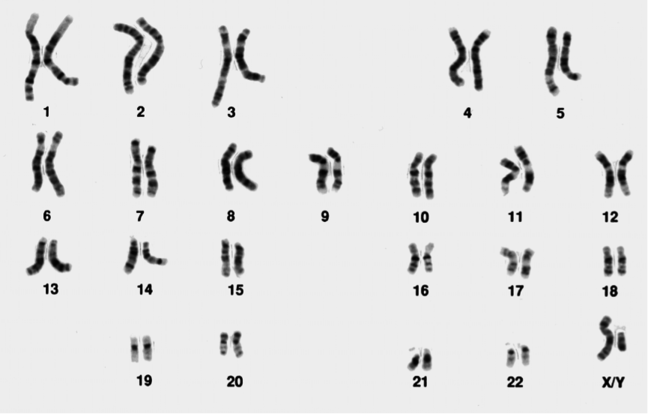
Figure 1: A normal male karyotype (note the X and Y sex chromosomes)
Original image from the National Institutes of Health and used under a public domain license.
Original image from the National Institutes of Health and used under a public domain license.
When clinical cytogenetic analysis started in the 1960s, solid staining was used, which meant that the different chromosomes were only recognizable by size and shape.
In the 1970s the visualisation of chromosomes improved due to the development of specific stains and enzyme digestion techniques, giving the chromosomes reproducible banding patterns. Different techniques were utilised by different countries.
For instance, France used Q-banding whereas the UK used a combination of enzyme digestion with trypsin and G-banding (see below). Using these techniques, each chromosome pair could be visualised with a unique pattern, resembling a bar code.
This not only helped cytogeneticists to recognise the different chromosomes, but also allowed them to see when parts of the chromosome were rearranged, deleted or duplicated. The most widely used stain is the Giemsa stain, giving the familiar G-banding appearance shown in Figure 1.
How is a karyotype done?
Karyotyping can be done from any sample from which you can obtain nucleated cells which retain the potential to divide, most commonly:
- Blood
- Bone marrow
- Skin
- Prenatal samples (such as CVS or amniotic fluid)
Cells are cultured in an environment which includes stimulants for cell division, then arrested at metaphase and harvested for analysis. Cell culture can take anything from 3 days (blood/bone marrow) to 7-14 days (skin, prenatal samples).
This requirement to culture the cells increases the turnaround time, but is unavoidable.
Once harvested, the cells are mounted on slides, banded (enzyme treated and stained), then viewed under a light microscope (x1000).
How is a karyotype analysed?
The cytogeneticist counts the chromosomes, pairs the homologues and compares the banding pattern in each set of homologues to identify any differences. They are aided by software which allows them to ‘cut and paste’ the chromosomes so that they can be aligned for comparison, as shown in Figure 1.
Many attempts have been made to develop computer software to replace the job of the trained cytogeneticist but it has not been possible to replace the pattern-recognition skills of the human eye.
Even in the best hands however, the limit of resolution of a karyotype is around 5 Mb, and in many applications karyotyping has been superseded by the use of higher-resolution techniques such as array-CGH.
What sorts of abnormalities can a karyotype identify?
Karyotyping is able to identify numerical abnormalities in the chromosomes (also known as aneuploidy), where a chromosome is missing or present in one or more extra copies. Examples of this include trisomy of chromosomes 13, 18 or 21, or sex chromosome abnormalities such as Turner syndrome (45,X) or Klinefelter syndrome (47,XXY).
Karyotyping can also identify structural abnormalities in the chromosomes including rearrangements (such as translocations, insertions or inversions), deletions and duplications.
Chromosome deletions can be detected on karyotype down to a resolution of around 5 Mb, but some are very subtle and require a trained eye.
The karyotype shown in Figure 2 shows a 22q11.2 deletion associated with velocardiofacial (or DiGeorge) syndrome. Would you have spotted it?
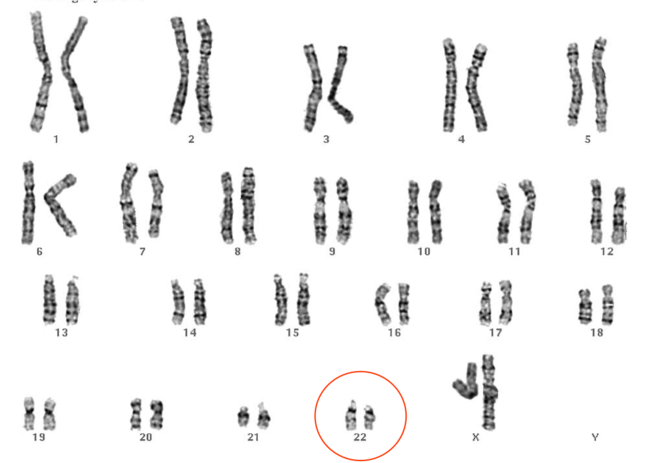
Figure 2: Karyotype showing 22q11.2 deletion
© St George’s, University of London
© St George’s, University of London
How are karyotypes reported?
A clinical karyotype report will include a description of the cytogenetic findings, described using standardized nomenclature and displayed using a cartoon representation of the chromosome and its banding pattern, called an ideogram (Figure 3).
Figure 3: Ideogram representation of chromosome 13 Original image from Human chromosome ideograms from NCBI’s Genome Decoration Page
There may also be a description of any additional tests undertaken to confirm the results, a literature review with any suspected associations with phenotype, a description of any follow-up studies required and, where appropriate, recurrence risks will be given.
What is karyotyping used for currently?
In many contexts karyotyping has been superseded by the use of array CGH, which can also pick up numerical and structural abnormalities in the chromosomes, and at much higher resolution, however karyotyping is still in use in certain clinical situations.
One such situation is in the investigation of infertility or recurrent miscarriage, where a balanced rearrangement is suspected. A balanced translocation describes a rearrangement of chromosomal material, but with no overall gain or loss of genetic material.
The balanced translocation carrier is usually unaffected themselves, however problems may arise when they try to have children as the balanced translocation can affect the way that the chromosomes divide during meiosis, and imbalance can then occur in the offspring.
In this context, karyotyping has a distinct advantage over array CGH in that it is able to detect balanced rearrangements, which array CGH is not.
This is because a karyotype provides a visual representation of the chromosomes, unlike array CGH, which provides quantitative information on the amount of chromosomal material present, but not its location.
Answers:-
1. D
An EDTA tube cannot be used. The correct blood tube to use for karyotype analysis is lithium-heparin. Samples for array CGH are collected in an EDTA tube however.
2. B
For karyotype preparation chromosomes are arrested in metaphase.
3. D
One copy of chromosome 22 looks shorter, and one copy of chromosome 9 appears longer. This translocation gives rise to an oncogenic fusion gene bcr-abl, causing chronic myeloid leukaemia (CML).
4. D
The short arm of the left-hand chromosome 4 is shorter in the top image than in the bottom image, due to a 4p deletion. This is associated with Wolf Hirschhorn, a developmental disorder characterised by a distinctive facial appearance, a (usually) severe intellectual disability and sometimes seizures.
Question 1
Answers:-
1. D
An EDTA tube cannot be used. The correct blood tube to use for karyotype analysis is lithium-heparin. Samples for array CGH are collected in an EDTA tube however.
2. B
For karyotype preparation chromosomes are arrested in metaphase.
3. D
One copy of chromosome 22 looks shorter, and one copy of chromosome 9 appears longer. This translocation gives rise to an oncogenic fusion gene bcr-abl, causing chronic myeloid leukaemia (CML).
4. D
The short arm of the left-hand chromosome 4 is shorter in the top image than in the bottom image, due to a 4p deletion. This is associated with Wolf Hirschhorn, a developmental disorder characterised by a distinctive facial appearance, a (usually) severe intellectual disability and sometimes seizures.
No comments:
Post a Comment